Acerand Therapeutics submitted an IND application for FGFR2 inhibitor ACE-16229210, its second candidate to enter clinical trials. The ACE-86225106 PARP1 inhibitor clinical trial enrolled its first patient in March 2024.
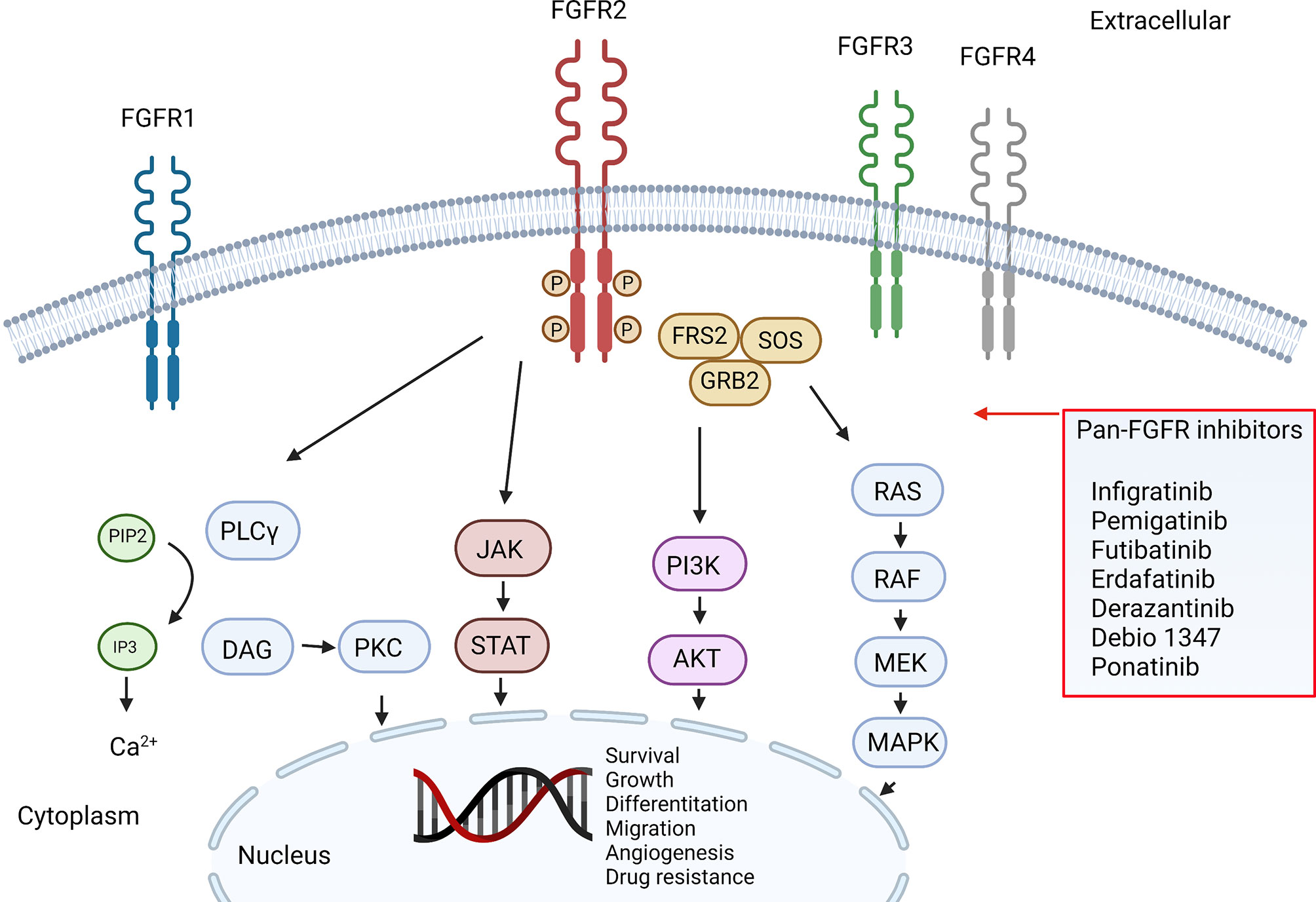 |
| FGFR pathway and inhibitors |
ACE-16229210
Recently, Acerand presented the preclinical results at the AACR Annual Meeting. ACE-16229210 potently inhibits FGFR2 (IC50 = 6.2 nM) with excellent selectivity (>133-fold) over FGFR1 and FGFR4. It also potently (IC50 <1 nM) and selectively (≥500-fold) inhibits FGFR2-induced ERK phosphorylation in multiple cancer cell lines harboring FGFR2 fusions, amplification, and mutations, but not those harboring FGFR1, FGFR3, or FGFR4 genetic alterations (IC50 > 460 nM).This molecule shows a robust broad spectrum of antitumor activity with significant tumor regression at low doses (1-10 mg/kg) in several tumor xenograft and PDX models representing the major FGFR2 relevant tumor histologies including gastric, breast, ovarian, and endometrial cancers harboring clinically important FGFR2 driver alterations.
In 2022, Acerand filed a patent (WO/2023/192502) covering spirobicyclic compounds that inhibit FGFR2. Acerand synthesized compounds modified from lirafugratinib. Selected compounds and lirafugratinib (developed by Relay) are listed below. Examples 18, 29, and 56 demonstrated an IC50 less than or equal to 1 nM. Recently, we reported initiating the clinical trial of 3HP-2827, another FGFR2 inhibitor discovered by 3H Pharma.
| Compounds in patent |
FGFR2 inhibitors
Selective FGFR2 inhibitors may avoid the severe on-target toxicity hyperphosphatemia and diarrhea associated with inhibition of FGFR1 and FGFR4. Phase 1 trial of the FGFR2/3 inhibitor KIN-3248 is undergoing. The 40 mg QD dose has been well evaluated, and the 50 mg QD dose is ongoing.
Lirafugratinib, developed by Relay Therapeutics, is currently in the phase 1/2 stage, with over one hundred patients already treated during its development.
In China, multiple FGFR inhibitors are currently in development. However, as of now, there is no specific selective FGFR2 inhibitor that has advanced to the clinical stage. The FGFR2 inhibitor JS122, co-developed by Junshi, remains in the preclinical stage. The planned Phase 1 trial aims to enroll a total of 60 participants globally, with 45 participants in China.
Comments