3H Pharma initiates the first-in-human study of 3HP-2827 in patients with solid tumors with FGFR2 alterations
3H (Suzhou) Pharmaceuticals (思康睿奇药业) commences its first-in-human study (NCT06287918) of 3HP-2827, an FGFR2 inhibitor, in patients with solid tumors exhibiting FGFR2 alterations in the United States. The clinical trial aims to enroll an estimated 130 participants.
3HP-2827
The Investigational New Drug (IND) application was submitted to the China Center for Drug Evaluation (CDE) on December 30, 2023. The IND has been approved for use either as monotherapy or in combination with chemotherapy or immunotherapy in patients with advanced cancers. Additional IND applications will be filed in East Asian countries.
The FGFR2 belongs to the FGFR family of tyrosine kinase receptors. The family consists of 4 genes that encode single-pass transmembrane receptors that bind to FGF on the extracellular domain. Ligand binding trigger a signaling cascade that may exercise several cellular functions, including cell survival. It is estimated that FGFR2 genomic alterations are present in around 10-15% of ICCA, most of them consisting of fusions, but also different aberrations can drive oncogenic transformation, such as mutations and amplifications, which may account for up to 3% of the cases.
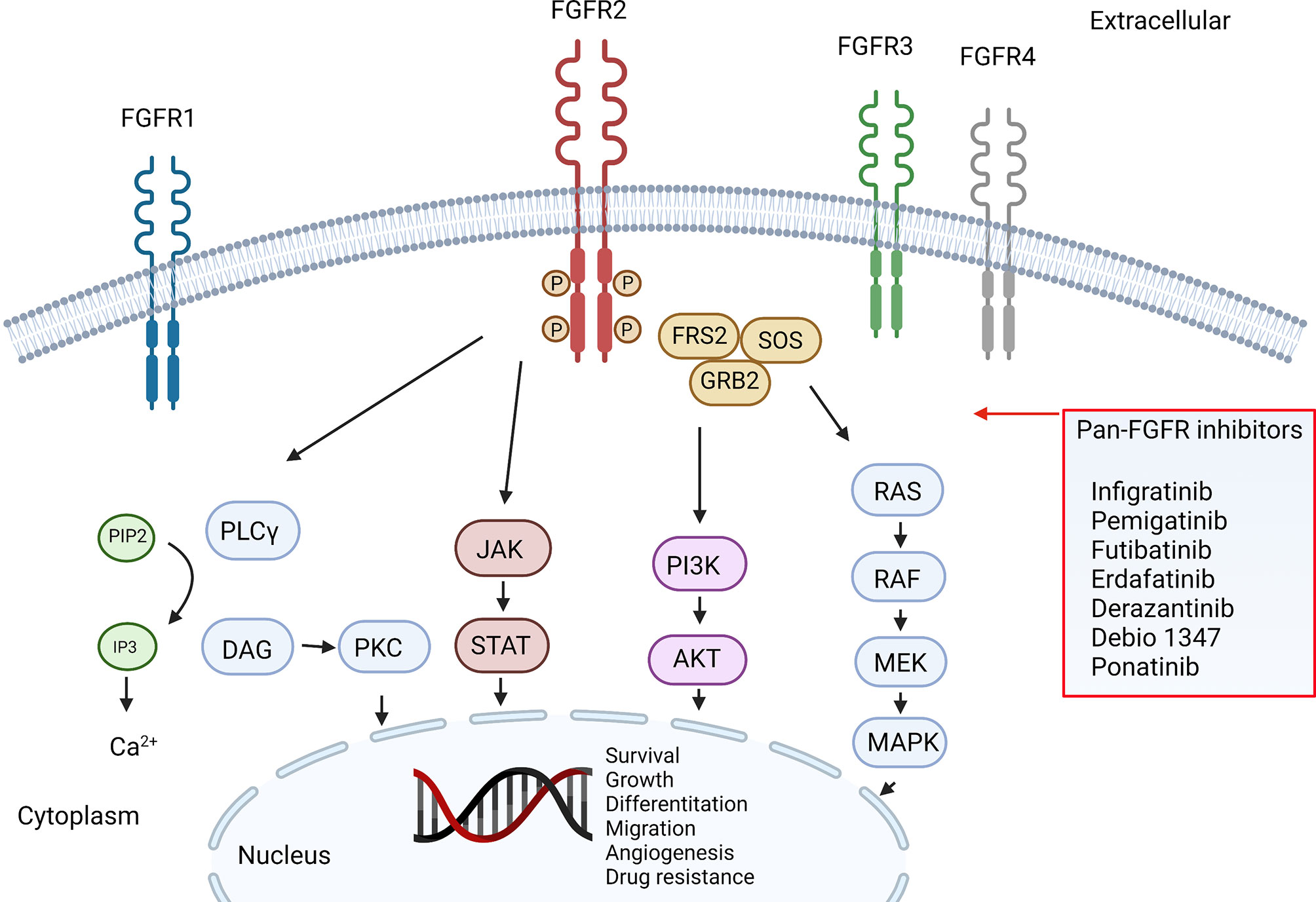 |
| FGFR pathway and inhibitors |
Cancers harboring FGFR2 gene fusions as well as those with FGFR2 amplification and/or FGFR2 activating mutations have demonstrated responses to pan-FGFR inhibition, however, the low rates and duration of responses suggest they were limited by toxicities.
A patent (WO/2023/046117) filed by 3H Pharma in 2021 covered FGFR2 inhibitors compared with Lirafugratinib by Relay. Here is the structure of compound 96 with an IC50 of less than 10 nm in an SNU16 cancer cell line.
| Examples |
FGFR2 inhibitors
Lirafugratinib, developed by Relay Therapeutics, is currently in the phase 1/2 stage, with over one hundred patients already treated during its development.
In China, multiple FGFR inhibitors are currently in development. However, as of now, there is no specific selective FGFR2 inhibitor that has advanced to the clinical stage. The FGFR2 inhibitor JS122, co-developed by Junshi, remains in the preclinical stage. The planned Phase 1 trial aims to enroll a total of 60 participants globally, with 45 participants in China.
Comments