Sintanovo, a company of Sun-Novo, has submitted the IND application for the peptide GHSR-1a agonist STC008. This is the second Class 1 new drug candidate, following the first clinical-stage STC007, a peptide KOR agonist for postoperative pain relief.
STC008
Growth hormone secretagogue receptor(GHS-R), also known as ghrelin receptor, a class A G protein-coupled receptor (GPCR), plays a diverse role in physiological functions, such as regulating appetite, alcohol intake, adipocyte metabolism, and maintaining glucose balance. GHSR1a, by binding with ghrelin, plays a significant role in stimulating appetite and increasing food intake.
 |
| The role of GHS-R is thought to be in regulating energy homeostasis and body weight |
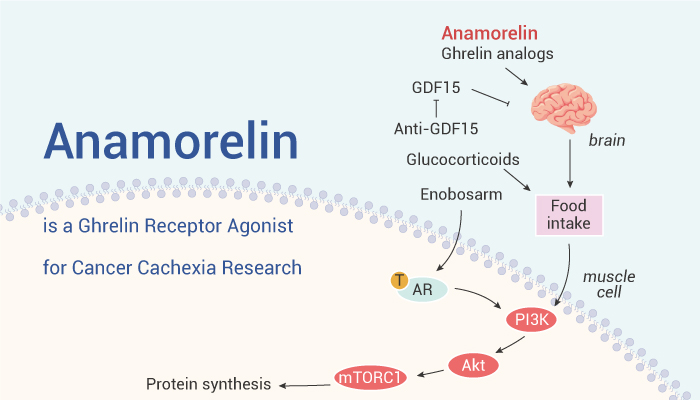
In 2020, anamorelin, a ghrelin receptor agonist, was approved as the world's first drug for the treatment of cancer cachexia in malignant tumors of non-small cell lung cancer, gastric cancer, pancreatic cancer, or colorectal cancer in Japan. Unlike megestrol or corticosteroids, anamorelin increases lean body weight rather than promoting fat accumulation.
In 2021, Sintanovo filed a patent (WO2022206587) covering the agonists of a growth hormone secretagogue receptor-1a (GHSR-1a). The polypeptide compounds disclosed in the patent exhibited agonistic activity for GHSR-1a, with an IC50 value of 0.3nM or higher. There was no data reported regarding the effects of these polypeptide compounds in animal models.
| Formula in patents |
GHSR agonists
As of now, Anamorelin is the only globally approved agonist for GHSR and the sole treatment available for cachexia; however, its approval is limited to Japan. The EMA rejected the marketing authorization for anamorelin, which was intended for the treatment of anorexia, cachexia, or unintended weight loss in patients with non-small cell lung cancer. This decision was based on findings from the ROMANA 1 and ROMANA 2 studies, which showed only a marginal effect of Adlumiz on lean body mass and no proven impact on hand grip strength or patients' quality of life. Following an inspection at clinical study sites, the conclusion was reached that a thorough evaluation of potential risks with anamorelin was not possible due to inadequately recorded safety data.
Helsinn presented the new results pf the global randomized, double-blind, placebo-controlled, multicenter phase 3 studies at the ESMO Congress 2023. Only one primary endpoint was met, while the other one failed to show significance. Anamorelin demonstrated a significant increase in body weight over a 12-week period compared to the placebo group (p<0.0001) in SCALA-1 and SCALA-2 trial. The least square mean treatment difference with a 95% confidence interval was +1.37 kg (+0.737; +2.001) for SCALA-1 and +1.30 kg (+0.720; +1.865) for SCALA-2. However, changes in the FAACT anorexia-cachexia 5-domain symptom score (5-IASS) scores for anamorelin versus placebo were not significantly different in either study.
As of now, Helsinn has not submitted a New Drug Application (NDA) to any new regulatory authorities around the world.
In 2021, Helsinn granted the exclusive license for anamorelin to Fosun Pharma in Mainland China, Hong Kong SAR and Macau SAR. However, there has been no reported progress or development since then.
Comments