Lepu Biopharma and Miracogen have submitted the IND application for MRG006A, an anti-GPC3 (Glypican-3) ADC in China, and the IND application is also expected to be submitted to the US FDA this year.
MRG006A
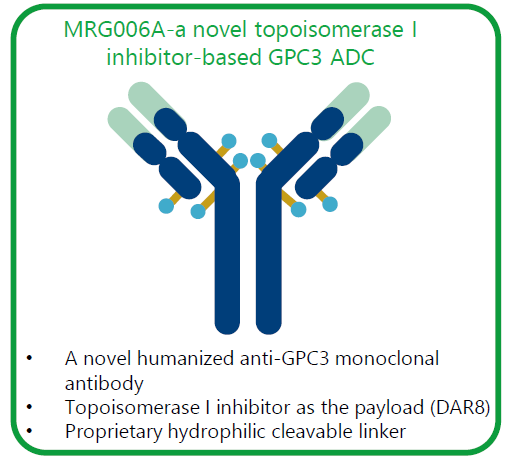
MRG006A is a GPC3-targeting ADC composed of a novel humanized IgG1 antibody conjugated to a potent topoisomerase 1 inhibitor via a peptide-based cleavable linker, with a DAR ratio of 8.
 |
| MRG006A |
Lepu Biopharma and Miracogen presented the results of the preclinical study of MRG006A at the 2023 AACR Annual Meeting. Highlights demonstrated its superior binding activity to GPC3-expressing cancer cell lines, rapid internalization, and potent GPC3-dependent cytotoxic activity.
In 2022, Miracogen filed a patent (WO2024061305) covering the GPC3 ADCs and their use in cancers, including liver cancer. The ADCs discussed in the examples included several candidates with DAR values ranging from 2 to 4, all of which were tested in the LIV#219 liver cancer PDX model.
GPC3 targeting ADCs
Currently, various products such as monoclonal antibodies, CAR-T therapies, and bispecific antibodies are being explored in early-phase clinical trials in patients with liver cancer and lung cancer.
Codrituzumab, an anti-GPC3 antibody developed by Chugai and Roche, was discontinued during the phase 2 study due to its failure to demonstrate clinical benefit in previously treated patients with hepatocellular carcinoma (HCC).
Several anti-GPC3×CD3 bispecific antibodies are in the early phase of development. Sanofi presented the preliminary results of the SAR444200 dose escalation study in patients with GPC3-positive hepatocellular cancer and non-small cell lung cancer last year. A grade 2 cytokine release syndrome was marked as a serious TRAE due to prolonged hospitalization in 8 patients dosed at two dose levels. CM350, a GPC3×CD3 bispecific antibody discovered by China-based KeyMed Bio, is still in the phase 1/2 study.
BC2027
BioCity Biopharma obtained the IND approval of the world's first GPC3 targeting ADC in the United States in April 2024. BC2027 is Biocity's second ADC product, following the anti-CDH3 ADC BC3195. In preclinical studies, BC2027 demonstrated anticancer activity with greater than 90% inhibition of tumor growth in some well-established cancers.
ZW251
The only reported anti-GPC3 ADC, ZW251, is conjugated with the Top1 inhibitor payload ZD06519 and has a DAR of 4 (a high value of 8 was not selected). This ADC was discovered by Zymeworks and is currently in the IND enabling stage, with plans to enter phase 1 study in 2025.
| ZW251 |
Comments