Kanghong Pharma has initiated the phase 1 clinical trial of KH801, a humanized anti-CD24 antibody, in patients with solid tumors. The China NMPA cleared the IND application of KH801 in March 2024. The first-in-human study was estimated to enroll 17-42 patients with 7 dose groups.
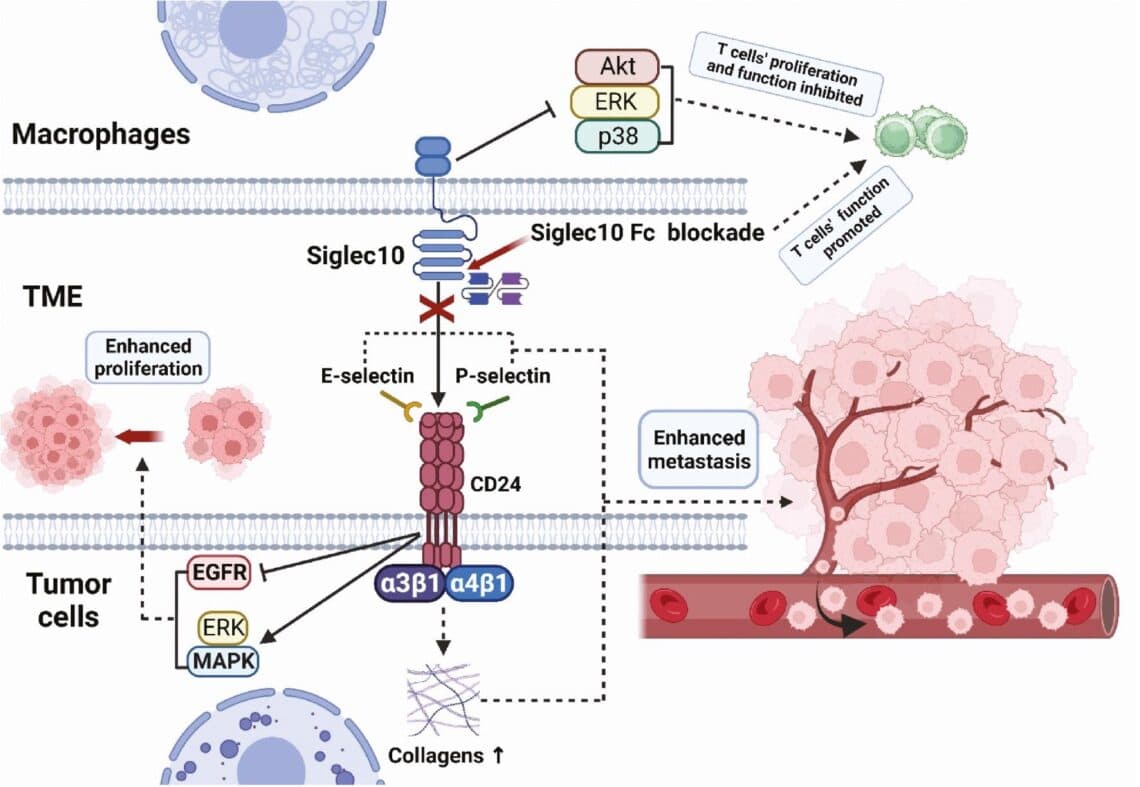 |
| Targeting the CD24-Siglec10 Axis |
KH801
KH801 eliminates tumor cells through Fc-mediated immune response and by inhibiting CD24/Siglec10 signaling in both. CD24, a small membrane protein heavily glycosylated and anchored by GPI, interacts with Siglec-10 on TAMs (Tumor-Associated Macrophages) to act as a 'don't eat me' signal, aiding tumor cells in evading macrophage engulfment. CD24 is prevalent across various solid tumors.
In 2021, Kanghong applied for a patent (WO2023104066) for using the anti-CD24 antibody or an antigen-binding fragment to treat tumors or immune diseases.
Kanghong presented the preclinical study results at the Society for Immunotherapy of Cancer's (SITC) 38th Annual Meeting. In comparison with competitor antibody, KH-801 owns more powerful binding potency to cancer cells and weaker binding affinity to human granulocytes, B cells, and T cells. KH-801 Fc variant 2 without binding to FcγRs, still promote phagocytosis of Monocyte-derived M2 macrophages. KH-801 promotes T cell infiltration into the tumor tissues and M2 TAMs elimination, that may transform cold tumors into hot tumors, and increase the anti-tumor potential for combination with other checkpoint inhibitors.
| KH801 vs ATG-031 in MC38-hCD24 mouse model |
Anti-CD24 antibodies
Enhanced "don't eat me" signals like CD47 help cancer cells avoid macrophage elimination, but their potent antibodies often cause red blood cell depletion. In contrast, CD24, another "don't eat me" signal, offers broader therapeutic potential with less toxicity due to its selective expression and absence on red blood cells. Currently, only two products (ATG-031, discovered by Antengene, and IMM47, discovered by ImmuneOnco) are in the clinical stage.
ATG-031
Antengene dosed the first patient with ATG-031 in December 2023, following the initiation of the first-in-human trial for patients with solid tumors and B-NHL in October 2023. During the dose escalation stage, 1 patient (n=5) achieved stable disease. The preliminary results were anticipated at the end of 2024.
Antengene posted the results of the preclinical study at the AACR23 Annual Meeting. ATG-031 treatment significantly inhibited the tumor growth of MC38-hCD24 on the right flank of mice with a tumor growth inhibition (TGI) of 60.52%. Interestingly, although the WT MC38 tumor on the left flank did not express the antigen, human CD24, ATG-031 still demonstrated efficacy in controlling the tumor growth with a TGI of 21.17%. Multiplex IHC staining revealed that the proportion of M1 macrophage increased in response to ATG-031 treatment, not only in MC38-hCD24 tumors but also in WT MC38 tumors on the left flank.
IMM47
ImmuneOnco developed IMM47, a humanized mouse anti-human CD24 mAb, and initiated a phase 1 trial in August 2023. The trial was estimated to enroll 48 patients with advanced solid tumors in six or more groups.
ImmuneOnco presented the preclinical results at the AACR23 Annual Meeting. At doses between 3 mg/kg to 10mg/kg, IMM47 significantly reduced tumor volume in a MC38 mouse model of colon cancer. Mice treated with 10mg/kg IMM47 produced tumor-specific immune response that prevented growth of re-inoculation of colon cancer cells. Further mechanism of action studies showed that IMM47 selectively and differentially increased M1 spleen macrophages quantity to 7-10 folds higher in that induced by PBS control. In contrast, IMM47 only increased M2 spleen macrophages to less than 1.5 folds higher than induced by PBS control. Expression of MHC II in M1 macrophages was also increased in IMM47-treated mice, suggesting significant activation and antigen presentation by macrophages. Our data has also confirmed that IMM47 specifically binds to and induces ADCC (antibody-dependent cellular cytotoxicity),ADCP (antibody-dependent cellular phagocytosis), and CDC (complement-dependent cytotoxicity) against a variety of cancer cells.
Comments