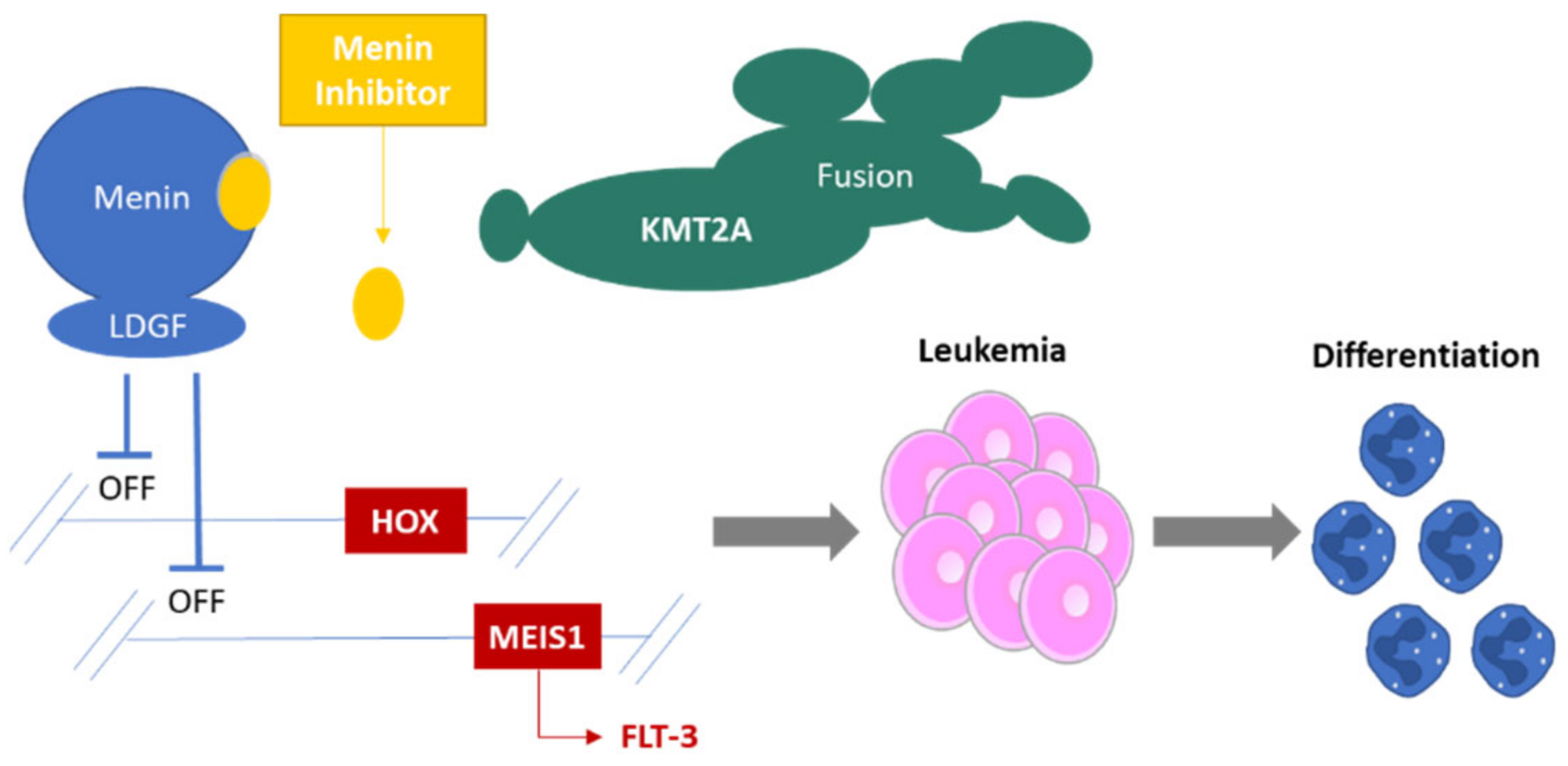
HUTCHMED (formerly Hutchison MediPharma) has introduced its Menin-MLL inhibitor HMPL-506 into a phase 1 trial, aiming to broaden its footprint in hematological malignancies. The company filed the IND application for HMPL-506 in January 2024 in China. Menin binds to KMT2A, activating leukemogenic genes like HOX and MEIS1. However, when a Menin inhibitor binds to the pocket of menin and displaces KMT2A, it deactivates HOX and MEIS genes, halting the growth of leukemic cells.
| Targeting Menin |
| HUTCHMED Pipeline in 2024Q1 |
HMPL-506
MLL gene rearrangements (MLL-r, also known as KMT2A) are found in 5%-10% of acute leukemias and are linked to a poorer prognosis. On the other hand, nucleophosmin 1 mutations (NPM1m) are the most frequent genetic changes seen in acute myeloid leukemia (AML). HMPL-506 is a menin-MLL inhibitor, as demonstrated by biochemical assays showing its potent blocking of menin-MLL binding with an IC50 of 1.0 nM. Compared to other Menin inhibitors in clinical stages such as SNDX-5613, KO-539, JNJ-75276617, and DSP-5336, HMPL-506 exhibited the most potent inhibitory effects in MLL-r and NPM1m cell line models.
| Biochemical assay IC50 |
In 2022, HUTCHMED submitted a patent (WO2024046457) that encompasses triazine compounds functioning as Menin inhibitors. The examples of compounds tested in the patent, alongside the reference compound revumenib, are as follows:
| Patent examples |
|
IC50 (μm) |
MV-4-11 GI50(μm) |
|
|
Example 110 |
0.0007 |
0.003 |
|
Example 119 |
0.0023 |
0.003 |
|
Example 151 |
0.0015 |
0.003 |
The first-in-human trial (NCT06387082) of HMPL-506 aims to enroll 98 patients, divided into three cohorts. These cohorts will consist of patients with MLL-rearranged and/or NPM1-mutant relapsed/refractory AML, MLL-rearranged relapsed/refractory ALL, and relapsed/refractory multiple myeloma (MM). Additionally, patients with AML exhibiting genetic alterations such as NUP214 or NUP98 fusion will also be included.
Menin inhibitors
The primary indication for Menin inhibitors, either alone or in combination with Bcl2 inhibitors, FLT3 inhibitors, or chemotherapy, in clinical studies is Acute Myeloid Leukemia.
Revumenib
Revumenib (SNDX-5613), discovered by Syndax, is the first Menin inhibitor to advance to the NDA stage. The FDA has granted priority review of the NDA for relapsed or refractory KMT2Ar acute leukemia (AML/ALL), with a PDUFA date set for September 26, 2024. The NDA for nNPM AML will be filed in the first half of 2025.
JNJ-6617
Janssen reported the results of the first-in-human phase 1 trial of JNJ-75276617 in patients with r/r AL harboring KMT2A or NPM1 alterations at the ASH Annual Meeting 2023. The combination with chemo or Bcl2 inhibitors is under evaluation in the phase 1 studies.
Ziftomenib
Kura Oncology aims to finalize enrollment for the registrational study of ziftomenib (KO-539) in patients with relapsed or refractory NPM1-mutated AML as monotherapy by mid-2024.
Balamenib
Eilean Therapeutics, which is developing multiple products for AML targeting FLT3 and BCL2, recently announced that they received IND approval for balamenib (ZE63-0302), a highly selective Menin inhibitor, in Australia last month.
DSP-5336
Sumitomo Pharma presented the preliminary results of the phase 1/2 study of DSP-5336 at the ASH Annual Meeting 2023. The registrational phase 2 trial is planned to start this year after discussions with regulatory authorities.
BMF-219
Biomea Fusion is currently investigating the safety and preliminary efficacy of BMF-219 in AML/ALL, following its initial target indication in diabetes. The dose escalation is estimated to be completed within this year.
HG153
HitGen, a company based in China, submitted the IND application for HG153 in September 2023 but has not received the implied IND approval even after the two-month deadline. As a result, it has returned to the IND filing stage.
Comments