China CDE has accepted for review a new drug application (NDA) seeking the approval of mufemilast, a PDE4 inhibitor developed by Hemay Pharma, for the treatment of plaque psoriasis. The NDA may be based on only one placebo-controlled randomized phase 3 trial, as the study was initiated before the approval of the standard therapy. The first PDE4 inhibitor, apremilast, was approved in China in 2021, which is 7 years after its approval in the United States.
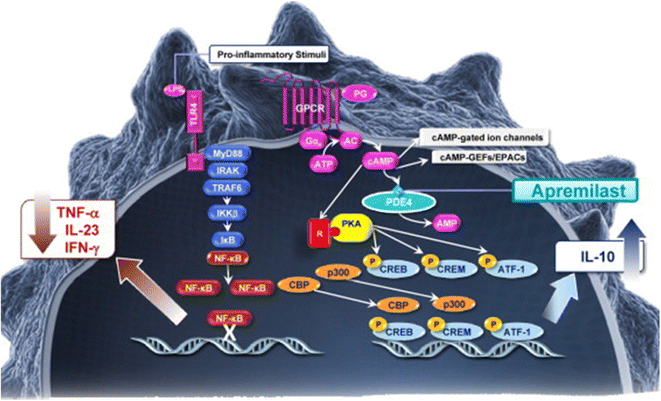 |
| PDE4 pathway |
Mufemilast
Mufemilast (Hemay005) is an orally active selective PDE4 inhibitor (in vitro IC50 = 80–120 nM), which is under evaluation in patients with plaque psoriasis, Behçet's disease, atopic dermatitis, ankylosing spondylitis, and ulcerative colitis.
| Hemay005 |
At ACR Convergence 2023, Hemay Pharma revealed the findings from clinical studies of mufemilast in patients with plaque psoriasis and Behçet's disease. In the phase 3 trial, mufemilast (60 mg BID) exhibited a PASI75 response of 53.6% compared to 16.0% in the placebo group among patients with moderate-to-severe plaque psoriasis at week 16. By comparison, apremilast (30 mg BID) demonstrated a PASI75 response of 33.1% and 28.8% versus 5.3% and 5.8% in the placebo groups across two phase 3 trials. Also for comparison, during the head-to-head studies with apremilast, the TYK2 inhibitor deucravacitinib exhibited robust efficacy in achieving PASI75, with rates of 58% and 53% compared to 40% and 35% in the apremilast groups.
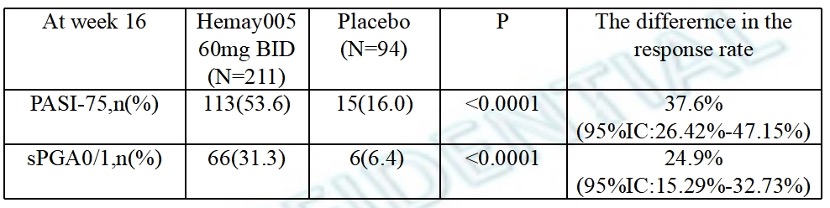 |
| Efficacy results |
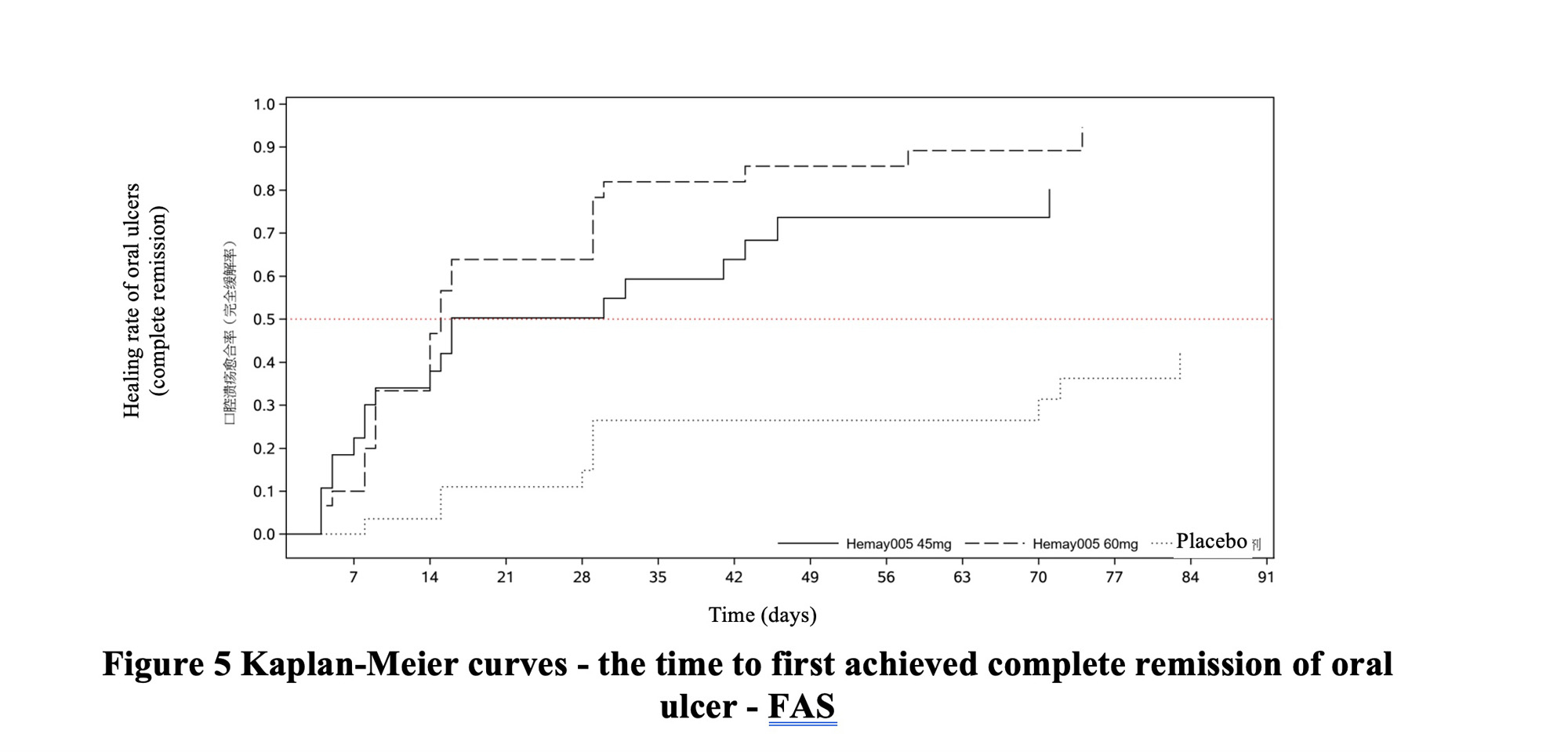 |
| Behçet's disease |
PDE4 inhibitors in China
Several China-based companies are developing PDE4 inhibitors, including some licensed-in products. For instance, roflumilast is being developed by Huadong under license from Arcutis, orismilast by Innovent under license from UNION/LEO, and HPP737 by Newsoara under license from vTv. They are primarily focused on the main indications of plaque psoriasis and atopic dermatitis, although some are considering exploring alternative pathways into COPD or IPF following potential efficacy signals or approval in other indications.
QY101, a PDE4 inhibitor ointment discovered by China-based E-nitiate Biopharma, is currently under evaluation for its effectiveness in treating psoriasis and atopic dermatitis in the phase 1 study.
Comments