CSPC Pharmaceutical is conducting a phase 2 study (CTR20241075) for nonsegmental vitiligo of SYHX1901, an oral JAK/SYK inhibitor. The first phase 2 study for psoriasis began in March 2023. The first-in-human study, initiated in healthy participants in 2021, has been completed in 2022. The study was aimed at patients with SLE and RA.
SYHX1901
SYHX1901 is a dual inhibitor of JAK and SYK, expected to be effective in treating autoimmune diseases such as psoriasis, lupus, and vitiligo.
In 2023, CSPC filed a patent (WO2022188796) covering the use of a compound containing a tricyclic heteroaryl group for the treatment of autoimmune disease.
| 2021 Patent |
The IC50 values for the compound I were measured against JAK1, JAK2, JAK3, TYK2, and SYK, yielding values of 20.04 nM, 3.92 nM, 1.43 nM, 0.82 nM, and 7.25 nM, respectively.
Efficacies for psoriasis, atopic dermatitis, and SLE were tested in the IMQ-induced model, OXA-induced model, and SLE model mice.
| Efficacy results in SLE model |
According to the toxicological study data, the single ascending doses of the phase 1 clinical trial to be carried out were 15 mg, 45 mg, 60 mg, 90 mg, 135 mg, 180 mg, 240 mg, 300 mg, and 360 mg. On the clinical study page, the prescribed doses were 7.5 mg QD and 30 mg QD. The final dose selected for patients with psoriasis and vitiligo in the phase 2 study was 30 mg QD.
JAK inhibitors in vitiligo
JAK inhibitors target the JAK/STAT pathway and are now approved to treat many immune-related diseases. In the treatment of vitiligo, JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, are effective, supporting the implication of the IFN-γ-chemokine signaling axis in the pathogenesis of vitiligo.
In 2022, the US FDA approved ruxolitinib cream, the first JAK inhibitor for treating nonsegmental vitiligo. F-VASI75 (at least 75% improvement in T-VASI) for the treatment group increased to ~30%, compared to 7.5%-12.9% for the placebo group.
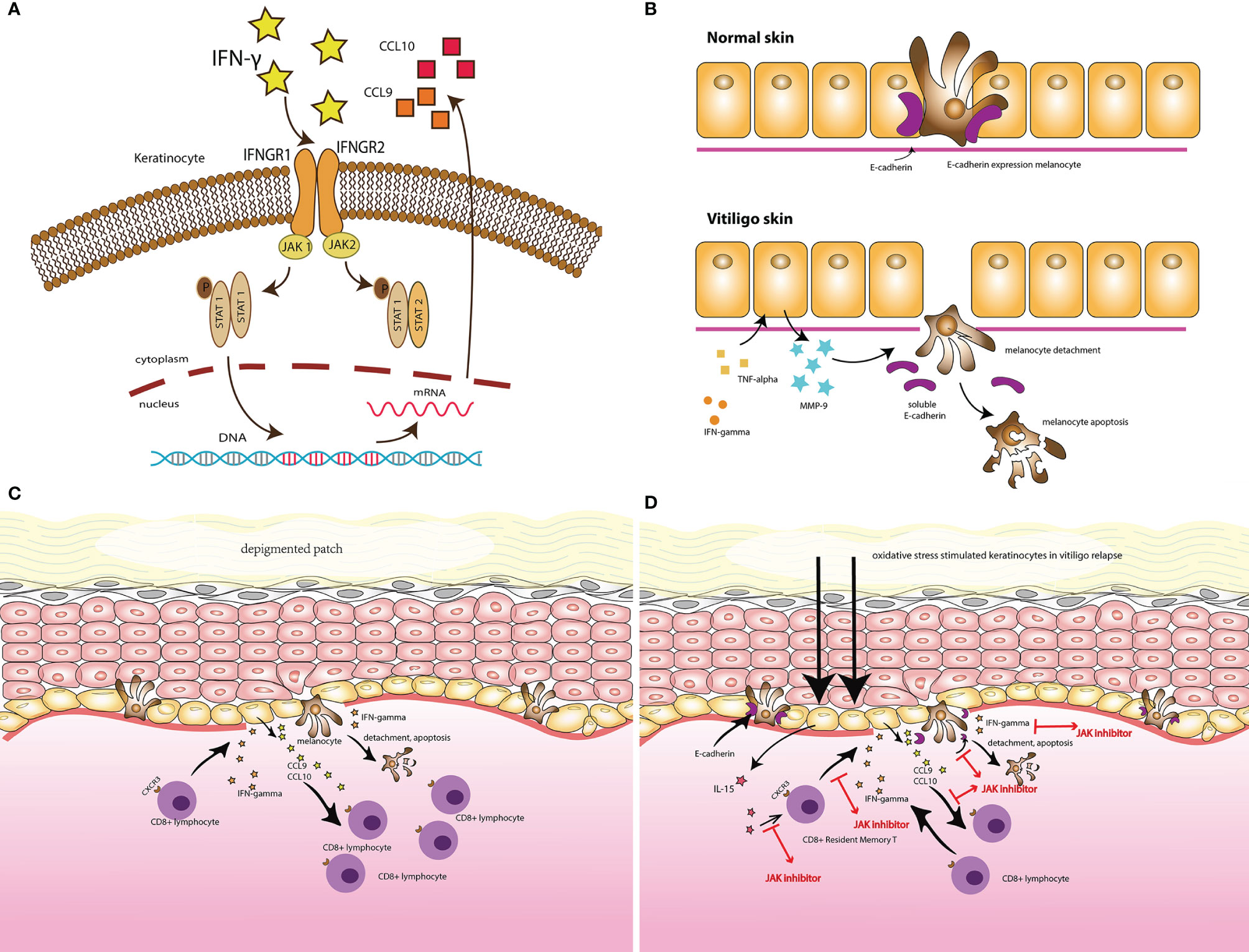 |
| The potential target for JAK inhibitors to treat vitiligo |
JAK/SYK inhibitors
Gusacitinib, developed by Asana Biosciences, was not further studied for autoimmune diseases after phase 2 trial results for atopic dermatitis in 2019. ASN002 was superior to placebo for the proportion of patients achieving EASI 50 (20 mg 20%, p=0.93; 40 mg 100%, p=0.003; 80 mg 83%, p=0.03; placebo 22%), EASI 75 (20 mg 0%, p=0.27; 40 mg 71%, p=0.06; 80 mg 33%, p=0.65; placebo 22%)
R333 and R348, developed by Rigel Pharma for cGvHD, dry eyes, and lupus, were discontinued after failing to reduce the redness and roughness of flaky skin in patients in recent decades.
CVXL-0255, a JAK3 and SYK dual inhibitor developed by Clevexel Pharma, is currently in the preclinical stage for immuno-inflammatory diseases. As described by Clevexel, the selectivity makes it possible to anticipate a better safety of use compared to other kinase inhibitors, targeting only Syk or one or more kinases of the JAK family.
Comments