On December 26th, 2023, Henlius filed the Investigational New Drug (IND) for HLX6018 (monoclonal antibody targeting the GARP/TGFβ1 complex) for the treatment of chronic inflammatory diseases in China.
As reported by Niklas Zimmer, "Regulatory T cells (Treg) play a critical role in immune homeostasis by suppressing several aspects of the immune response. Herein, Glycoprotein A repetitions predominant (GARP), the docking receptor for latent transforming growth factor (LTGF-β), which promotes its activation, plays a crucial role in maintaining Treg mediated immune tolerance. After activation, Treg uniquely express GARP on their surfaces. Due to its location and function, GARP may represent an important target for immunotherapeutic approaches, including the inhibition of Treg suppression in cancer or the enhancement of suppression in autoimmunity.
In 2022, Henlius submitted a patent (WO/2022/116877) for an anti-GARP/TGFβ antibody and its method of use. The binding ability was reported in the examples.
"The antibodies' binding ability to human GARP and human GARP/TGFβ1 complex was further tested by Octect. Unlike GA1#8 and ABBV-151, which only bound to GARP/TGFβ1 complex, hGA17 bound to both GARP/TGFβ1 complex and GARP with comparable affinity. DS-1005a analog was capable of binding to GARP/TGFβ1 complex and GARP, but the binding was much weaker compared to hGA17."
"hGA17 inhibited mature TGFβ1 release from thrombin-activated human platelets at a dose of 50 μg/ml. GARP ref. Ab1 showed a similar pattern of inhibition TGFβ1 release from thrombin-activated human platelets, however, the inhibition effect of GARP ref. Ab2 was not observed in this assay."
"hGA17 induced strong cytotoxicity in a dose-depend manner, while other anti-GARP/TGFβ antibodies only induced weak or no cytotoxicity towards Hs 578T cells. The superior ADCC effects of hGA17 indicated that hGA17 can have improved anti-tumor efficacy in tumors having high GARP/TGFβ complex expression."
"hGA17 reduced GARP +Treg population in four different donors to a greater degree than all other anti-GARP/TGFβ antibodies. The results indicated that hGA17 had superior Treg depletion activity compared to other anti-GARP/TGFβ antibodies and can improve antitumor efficacy by reducing Treg population in the tumor microenvironment."
"both hGA17 and GA1#8 showed antitumor efficacy in the human GARP KI MC38 mouse model. The tumor growth inhibition (TGI) of hGA17 and GA1#8 was 45.81% and 38.55 %, respectively, compared to the vehicle control group on day 24. In contrast, on day 24, GARP ref. Ab1 showed much less tumor growth inhibition (TGI=16.57%), whereas GARP ref. Ab2 treatment did not show tumor inhibition compared to the vehicle control group."
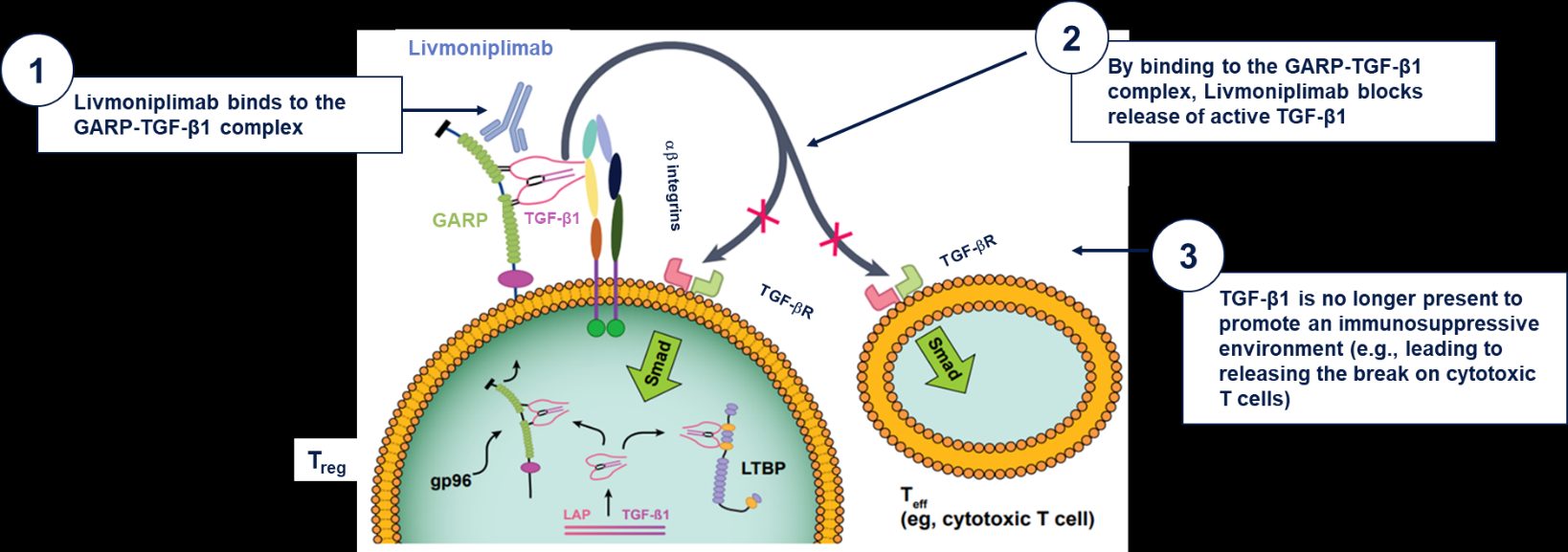
Livmoniplimab (ABBV-151; ARGX-115), a humanized monoclonal antibody developed by argenx and AbbVie, blocks the LRRC32-mediated activation of latent TGFβ1, a protein complex involved in tumor growth and immune suppression. When bound to the GARP-TGF-β1 complex, Livmoniplimab prevents the release of active TGF-β1. This inhibition of TGF-β1 signaling has the potential to improve the responsiveness to anti-PD1 therapy by aiding in the restoration of effector CD8+ T-cell activation, expansion, and infiltration within the tumor. This makes it a potential candidate for cancer research.
In April 2023, AbbVie began the first registrational trial (NCT05822752) of Livmoniplimab in combination with the anti-PD-1 mAb Budigalimab second-line HCC treatment following anti-PD-1 therapies.
In October 2023, AbbVie commenced a phase 2/3 trial (NCT06109272) evaluating Livmoniplimab in combination with the anti-PD-1 mAb Budigalimab in patients with untreated HCC, comparing it with Durvalumab and Tremelimumab.
 |
| Adapted from Metelli A, et al. Journal of Hematology & Oncology (2018) 11:24. http://creativecommons.org/licenses/by/4.0/ for CC BY 4.0 |
DS-1055, a monoclonal antibody specifically designed to target GARP developed by Daiichi Sankyo, aims to enhance antitumor immunity by depleting GARP-positive Tregs. Currently, it is in the Phase 1 stage of development (NCT04419532).
Comments